Skynor Medical announces Peripheral Vascular Hydrus® Balloon Dilatation Catheter System has been approved by the European CE certification Authority. The system is expected to be used for percutaneous transluminal angioplasty (PTA) for peripheral arteriovenous vascular occlusion or stenosis, for native or synthetic arteriovenous dialysis fistula occlusion, and post-dilation of balloon-expandable and self-expanding stents in the peripheral vasculature.
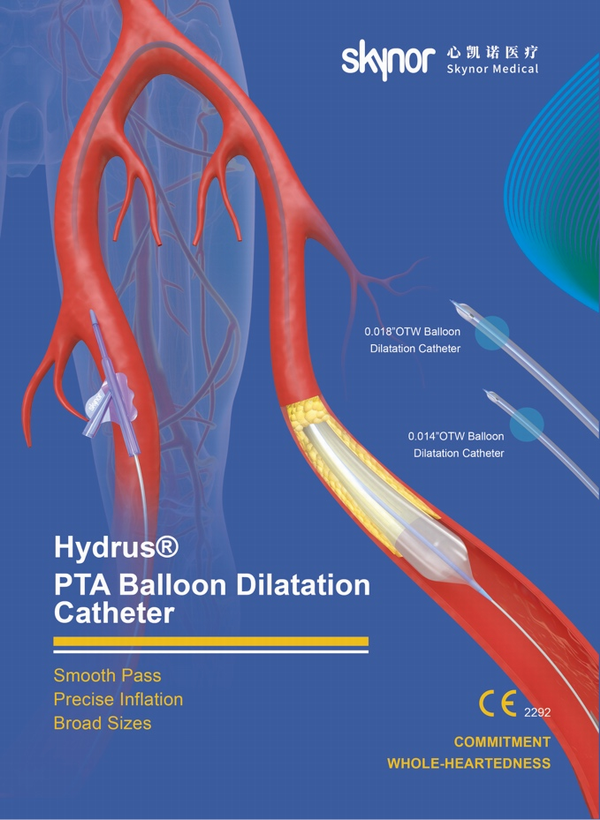
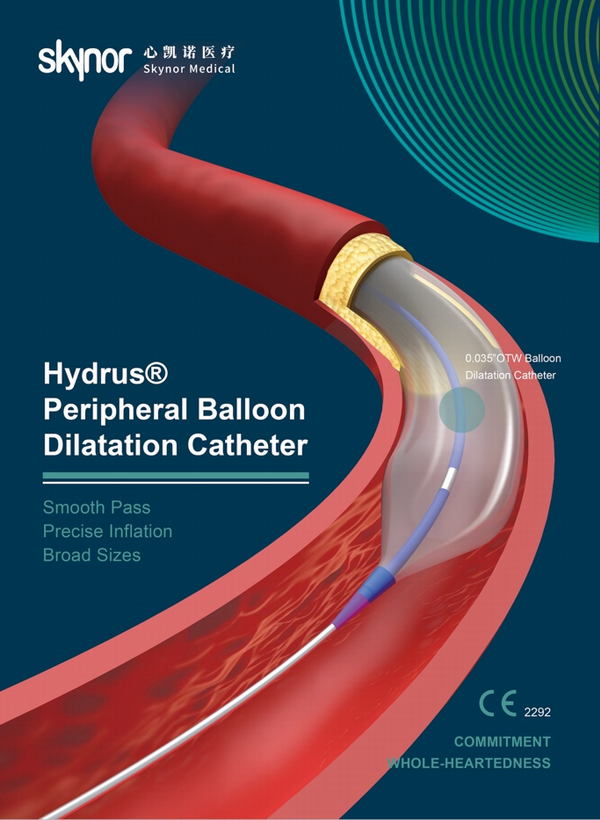
The Hydrus® portfolio is divided into three product lines: 0.014 "guide wire series, 0.018" guide wire series and 0.035 "guide wire series. With clinic benefits of pass lesions smoothly and efficiently , inflate stenosis precisely and broad sizes for more procedural options.
The Hydrus® Balloon Dilatation Catheter (NMPA 20213030015) and the Hydrus® PTA Balloon Dilatation Catheter (NMPA 20213030046) have been approved for launch in China since January 2021.
Post time: May-25-2021
