-
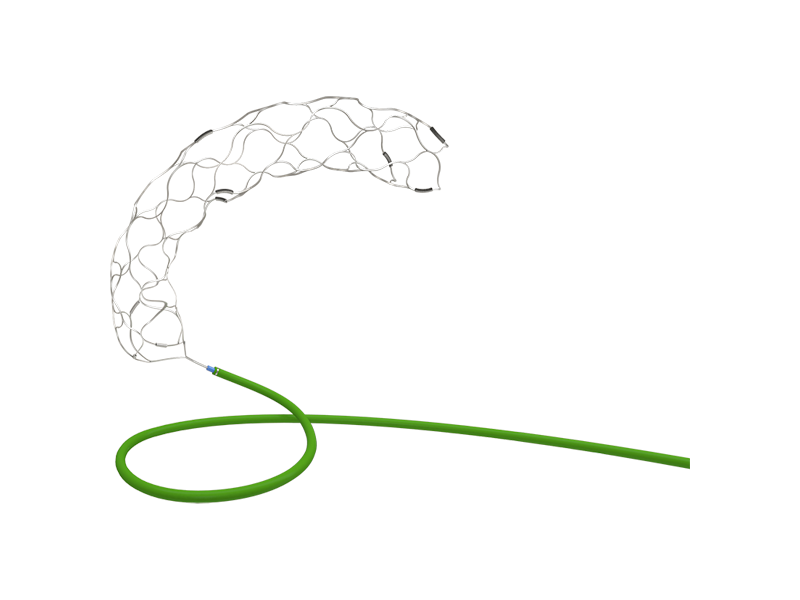
SkyFlow ® Thrombectomy Device
Indication:
Acute ischemic stroke caused by large artery occlusions.
-
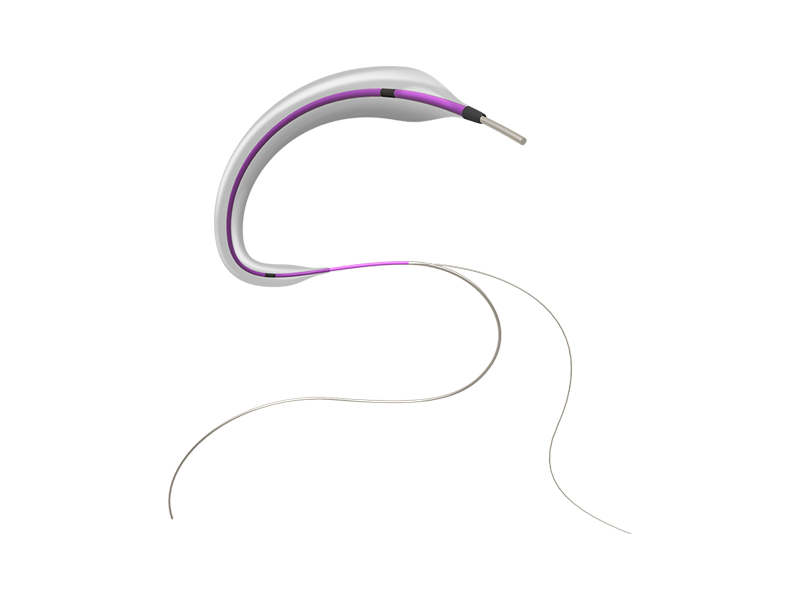
SkyGuard ® Balloon Guide Catheter
Indication:
This catheter is indicated for use in facilitating the insertion and guidance of an intravascular catheter into a selected blood vessel in the neuro vascular systems. The balloon provides temporary vascular occlusion during these and other angiographic procedures. The balloon catheter is a conduit for delivering and retrieving devices. -
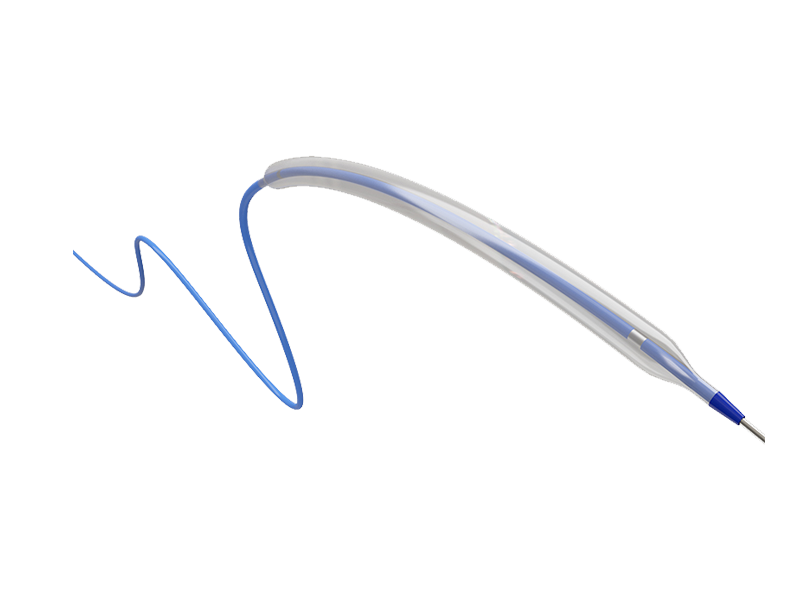
SkySurfer ® Distal Access Catheter
Indication:
This product is used to introduce interventional or diagnostic instruments into the cerebral and peripheral blood vessels. It can also be intended to aspiration thrombosis and debris from cerebral or peripheral arteries. -
SkyLoach ® Intracranial Balloon Dilatation Catheter
Indication:
This product is indicated for balloon dilation of the stenotic portion of intracranial arteries to improve intracranial perfusion. -
SkyGuard ® Balloon Guide Catheter
Indication:
This catheter is indicated for use in facilitating the insertion and guidance of an intravascular catheter into a selected blood vessel in the neuro vascular systems. The balloon provides temporary vascular occlusion during these and other angiographic procedures. The balloon catheter is a conduit for delivering and retrieving devices. -
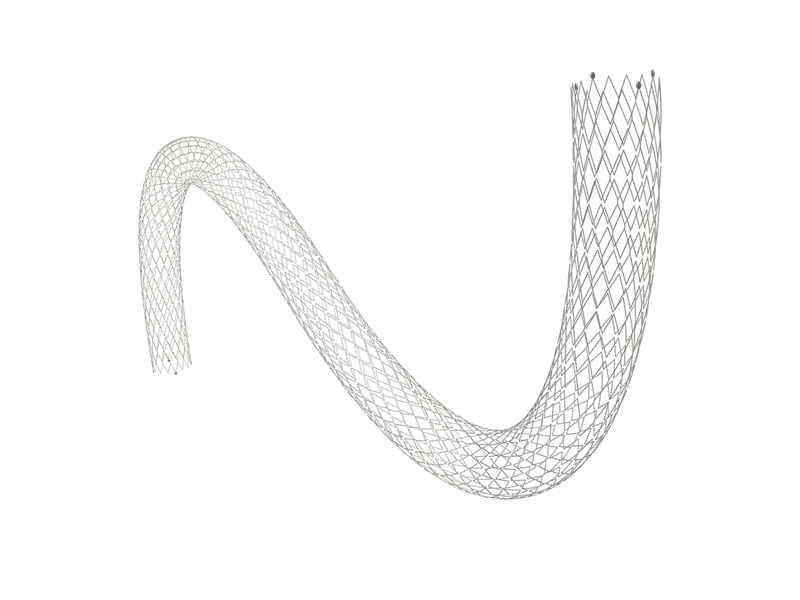
SkyNova ® Peripheral Vascular Stent System
Indication:
This product is intended for the treatment of peripheral vascular stenosis and occlusion of iliac artery and femoral artery of lower limbs. -
Hydrus ® PTA Balloon Dilatation Catheter
Indication:
The device is expected to be used for percutaneous transluminal angioplasty (PTA) for peripheral arteriovenous vascular occlusion or stenosis, for native or synthetic arteriovenous dialysis fistula occlusion, and post-dilation of balloon-expandable and self-expanding stents in the peripheral vasculature. -
SkyDragon™Thrombus Aspiration Catheter
Indication:
The product is intended for revascularization in patients with acute ischemic stroke secondary to intracranial large vessel occlusion (internal carotid artery, middle cerebral artery – M1 and M2 segments, basilar artery and vertebral artery) within 8 hours of symptom onset. Patients who cannot use intravenous tissue plasminogen activator (IV t-PA) or who have failed IV t-PA are candidates for this therapy. -
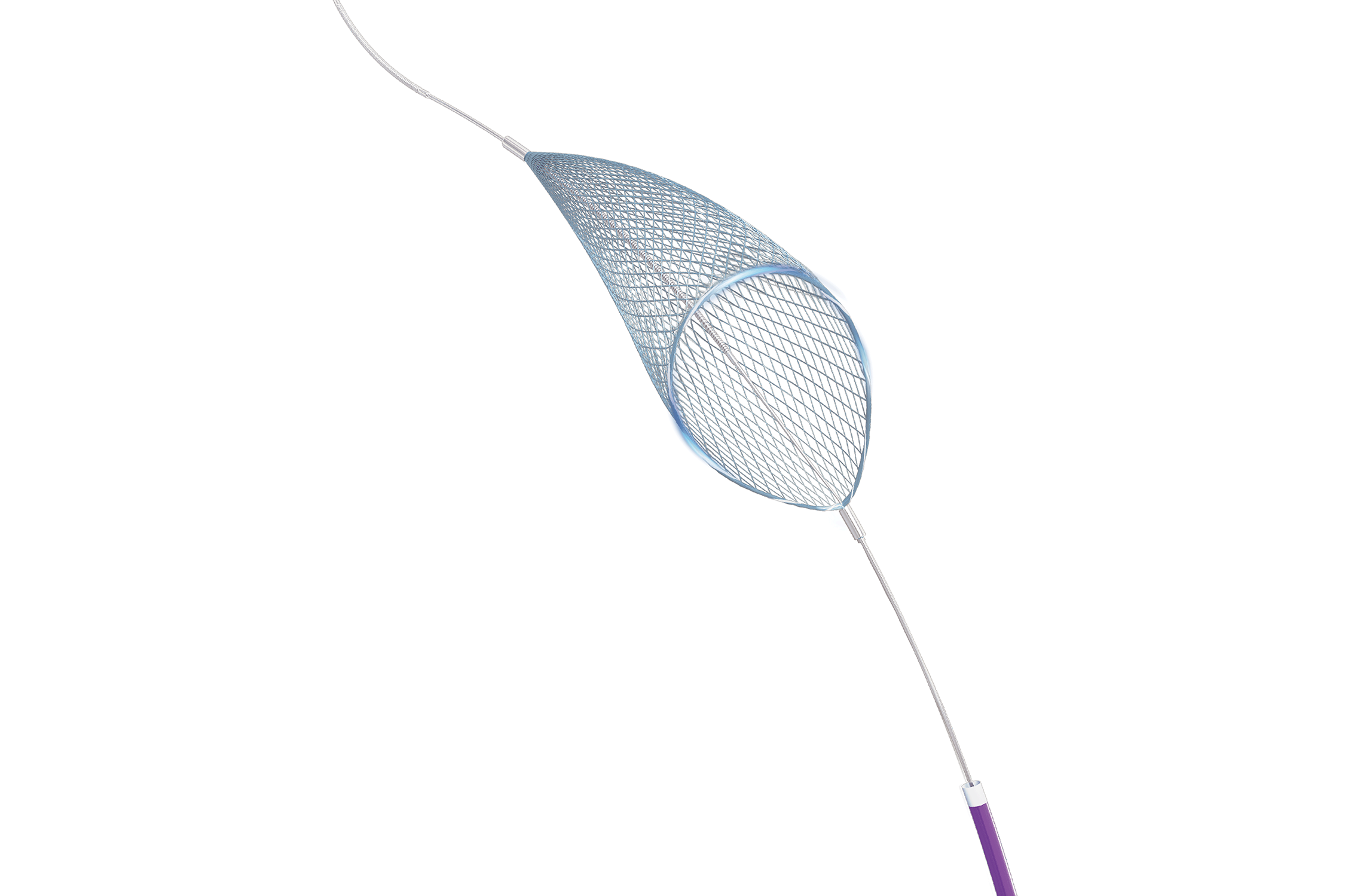
SkyKeeper ® Embolic Protection Device
Indication:
Embolic protectors are used to protect patients from distal embolization during vascular interventions, including peripheral vessels and carotid arteries.
-
SkySurfer ® Distal Access Catheter
Indication:
This product is used to introduce interventional or diagnostic instruments into the cerebral and peripheral blood vessels. It can also be intended to aspiration thrombosis and debris from cerebral or peripheral arteries. -
SkyManna ® Infusion Catheter
Indication:
The product is suitable for the liquid administration of thrombolytic agents and contrast agents into peripheral blood vessels.
-
SkyWay ™ Microcatheter
Indication:
The product is intended for the introduction of non-liquid interventional device (such as stents/flow diverters) and infusion of diagnostic (such as contrast media), or therapeutic agents into the neuro, peripheral and coronary vasculature.

 Acute Ischemic Stroke (AIS)
Acute Ischemic Stroke (AIS) Acute Hemorrhagic Stroke (AHS)
Acute Hemorrhagic Stroke (AHS) Access series
Access series Peripheral arterial disease (PAD)
Peripheral arterial disease (PAD) Peripheral venous disease (PVD)
Peripheral venous disease (PVD)