Solutions
Interventional treatment solutions against stroke and peripheral vascular disease for patients around the world
About Us
Skynor Medical is an innovative company focusing on R&D of state-of-the-art vascular interventional devices.
We’re committed to providing comprehensive interventional treatment solutions against stroke and peripheral vascular diseases for patients in China and other countries.
Products
News Center
-
Skynor Medical Announces the Approval of ISO13485 :2016quality management system certification
Skynor Medical Technology (Shanghai) Co., Ltd. announces the approval of the ISO 13485:2016 international system certification, and the obtainment of the international quality management system standard certification issued by TUV SUD group. ISO 13485 Medical devices -Qu...
-
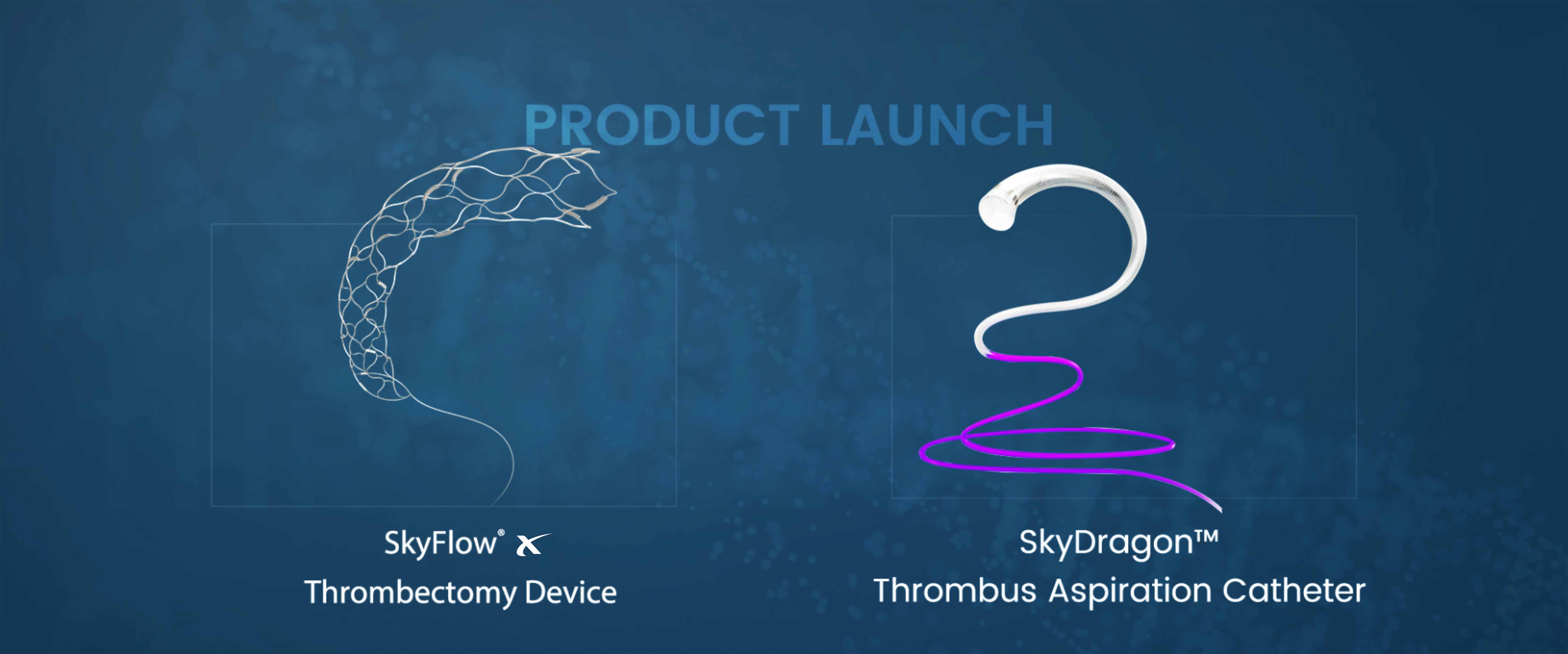
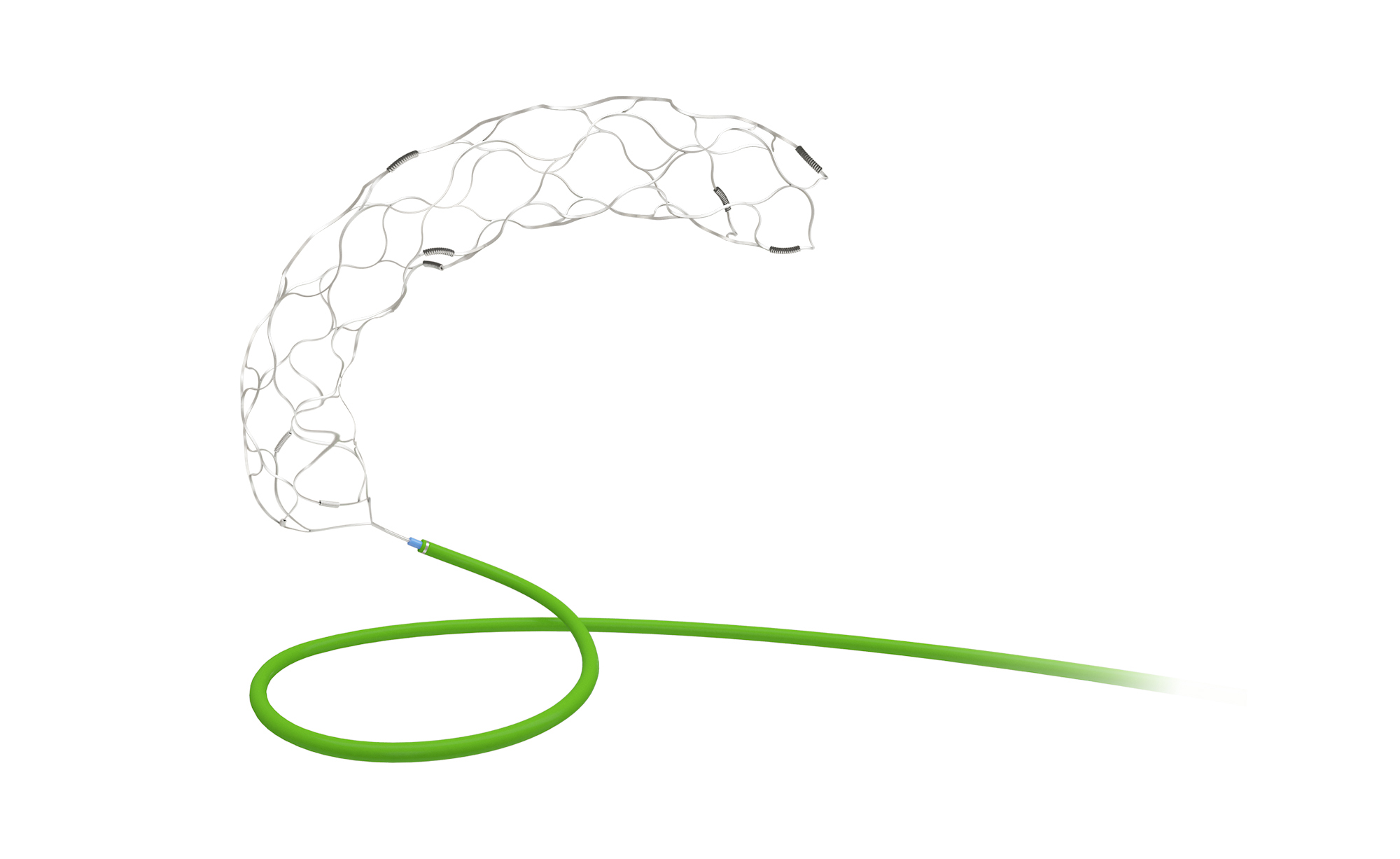
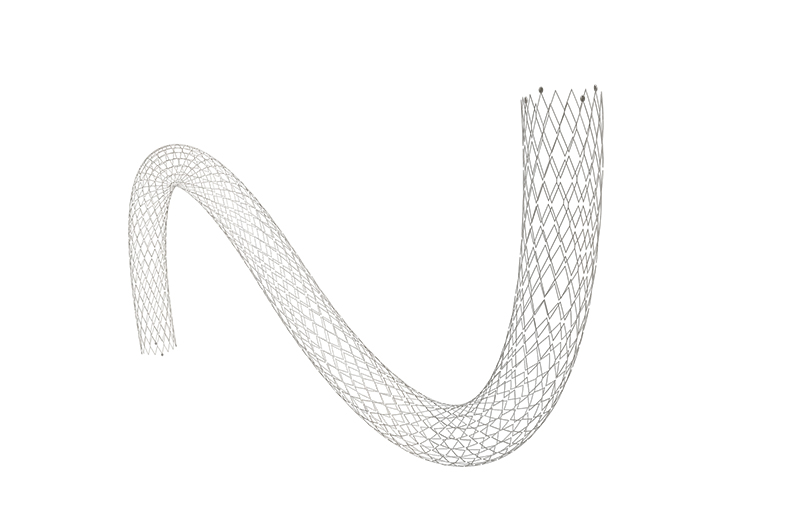
Skynor neurointervention portfolio to treat ischemic stroke all get CE mark
This system provides optimal treatment solution for Acute lschemic stroke patients worldwidely.
-
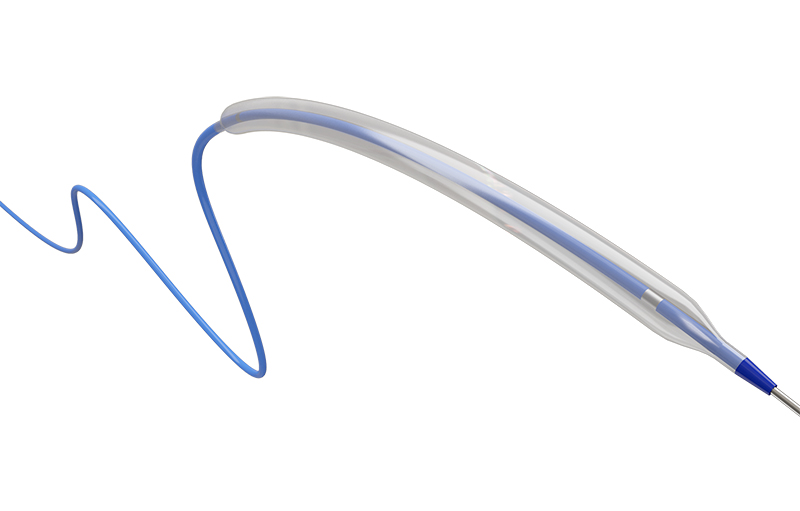
Skynor peripheral balloon dilatation catheter Hydrus gets CE mark
Skynor Medical announces Peripheral Vascular Hydrus® Balloon Dilatation Catheter System has been approved by the European CE certification Authority. The system is expected to be used for percutaneous transluminal angioplasty (PTA) for peripheral arteriovenous vascular o...